Recall On Eye Drops October 2025
Recall On Eye Drops October 2025. Four eye ointment products have been recalled due to a potential risk of eye infections, the food and drug administration (fda) shared monday. The indian manufacturer of eye drops linked to three deaths and serious infections in the us violated several safety norms, the country's top regulator has said.
The full list is here, and it includes eye drops made by one manufacturer and. Eye drops maker kilitch healthcare india has recalled 27 eye drops that pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defences, the usfda said.
The fda has expanded its list of eye drops recalled in 2025 because the products could be tainted with bacteria.

Food and drug administration on friday warned consumers to not purchase or use certain eye drops from several brands, including cvs health corp and cardinal health,.

FDA Recalls NDC 49348037 Sunmark Eye Drops Original Formula Liquid, Look up any otc eye drop sold in the united states for recalls and red alerts. Last updated on dec 21, 2025.

Eye drops recall FDA warns to immediately stop using 26 types of over, An earlier recall of potentially contaminated eye drops has been expanded to more than two dozen products sold at major retailers including cvs, target, rite aid, walmart and others. The recalled eye ointments, manufactured by.
Several eye drops and ointment sold at Walgreens and Walmart recalled, Implicated in the warning letters issued in october 2025, kilitch healthcare india has voluntarily recalled eyedrops distributed to retailers nationwide. The fda has expanded its list of eye drops recalled in 2025 because the products could be tainted with bacteria.

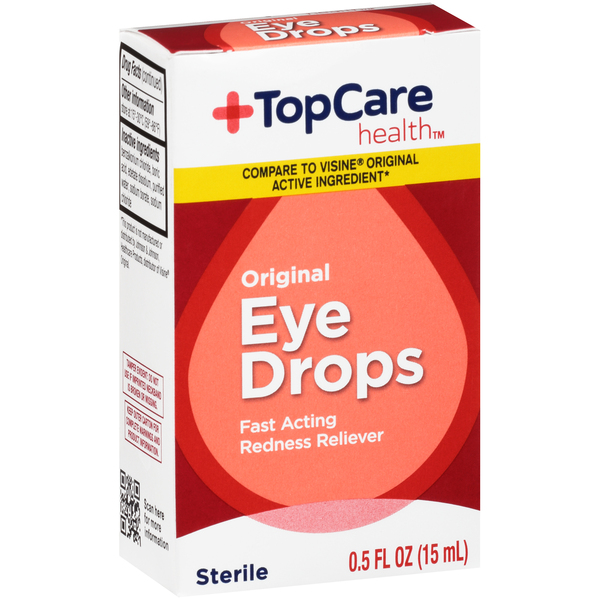
TopCare Eye Drops Recall, The recalled eye ointments, manufactured by. An earlier recall of potentially contaminated eye drops has been expanded to more than two dozen products sold at major retailers including cvs, target, rite aid, walmart and others.

Which eyedrops are recalled and why are they dangerous?, Indian manufacturer kilitch healthcare india limited voluntarily recalled 27 of its eye drop products that were distributed to stores including cvs, rite aid and target after the food and drug administration found insanitary conditions at its facility. Look up any otc eye drop sold in the united states for recalls and red alerts.

EzriCare recalls eye drops linked to infections YouTube, Food and drug administration on friday warned consumers to not purchase or use certain eye drops from several brands, including cvs health corp and cardinal health,. What you need to know.
Eye drop recall 2025 FDA finds sterilization issues at EzriCare, Implicated in the warning letters issued in october 2025, kilitch healthcare india has voluntarily recalled eyedrops distributed to retailers nationwide. Look up any otc eye drop sold in the united states for recalls and red alerts.

More than 10 different brands of eye drops recalled over contamination, Look up any otc eye drop sold in the united states for recalls and red alerts. The fda warned consumers in late october not to buy or use the products, saying they pose a risk of eye infections that could cause partial vision loss or blindness.

Global Pharma recalls eye drops after US flags 55 cases of adverse, The fda has expanded its list of eye drops recalled in 2025 because the products could be tainted with bacteria. The us food and drug administration (fda) has announced a nationwide voluntary recall of 27 eye drop products, including those sold at cvs, rite aid, and target, for potential safety reasons, including eye infection risk.
Eyedrop Recall Here’s Which Brands Have Been Linked to Infections, As of november 15, 2025, the manufacturer has officially recalled those eyedrops. Recalled artificial tear eye drops, gels, and ointments had previously been sold in retail stores, such as cvs and walmart, and online.
If pathogens from contaminated eye drops are applied directly to the eye, the resulting infection could also lead to vision loss and even blindness, the fda warns.